THNOECLOGY
1)Carbonation, Graphitization by former solid reaction
Artificial graphite,Carbon fiber,Activated carbon,Glass-like carbon of former carbon material and graphite material have been produced by performing a long-time heat treatment at high temp. around 1,000℃ to 3,000℃ on solid raw materials as oil/residual coal/organic matter.
Because of this, obtaining the high crystal characteristic material was difficult, light-weight (about half of aluminum) of carbon material, high strength(about 20times of steel iron), high current density tolerance(over 1,000times of copper) and thermal conductivity(about10times of copper), these excellent characteristics did not actualize and used to be materials having many crystal defects and structural defects
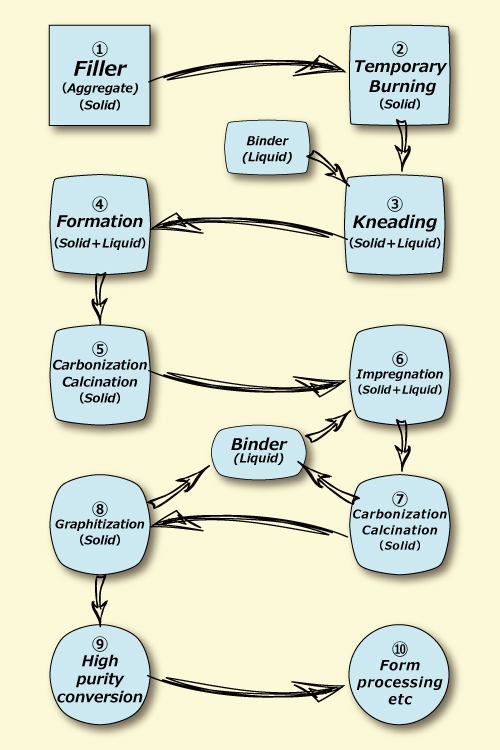
Fig. 1. Conventional manufacturing methods for carbon materials and graphite materials [case of artificial graphite]
2)Carbonization and graphitization through conventional vapor-phase reaction
In the manufacturing method of carbon materials through vapor-phase growth using gaseous raw materials such as hydrogen, carbohydrate, and argon, CVD reaction proceeds due to hydrocarbon radicals exited by heat, plasmas, or ions. Thus, the material with high crystallinity has been successfully obtained at around 1000°C of relatively low temperature, compared with solid-phase reaction. In the manufacturing method through vapor-phase growth, carbon nanotube, carbon nanofiber, etc. are synthesized using a metal catalyst such as iron, cobalt, or nickel. In the presence of a substrate, graphene, diamond-like carbon, etc. are obtained. In these vapor-phase growth reactions, carbon materials near single crystal can be obtained. In carbon tube, in particular, the excellent material characteristics that carbon materials have are expressed, which encourages active research and development.
3) Formation of carbon through conventional sublimation process
Fullerene and carbon nanotube have been synthesized by sublimating solid carbon at a high temperature at 4000°C or above based on arc discharge using solid carbon electrodes. In this method, there is an advantage to be easily able to obtain monolayer carbon nanotube. However, the yield of each material is low, and selective extraction process from the products is required.
4) Synthetic method of graphene through conventional method
Graphene, which is composed of a piece of carbon’s hexagonal net plane, was firstly separated by A.K. Geim and K.S. Novoselov. It was prepared by patching a Scotch tape to HOPG (highly oriented pyrolytic graphite) followed by peeling it. Its excellent characteristics have been clarified.
Fig. 2 Comparison between conventional CNT manufacturing method and InALA process
Table 1 shows the comparison between conventional carbon material manufacturing methods and InALA process with a focus on generable carbon materials, productivity, and purity.
InALA method can produce a high purity nano carbon with high productivity, compared with other manufacturing methods. As for graphene, in particular, this process can directly synthesize it without substrate and without catalyst.
Table 1 Comparison of manufacturing methods
After that time, several methods have been developed: a method to reduce again the graphite oxide that was once synthesized by Hummers method, a method to form a film based on CVD using a substrate like copper, and a method to sublimate Si on the surface of monocrystal SiC.
5) Problems of conventional synthetic methods of nano carbon
Carbon materials with the characteristics near monocrystal carbon or graphite can be synthesized by the above synthetic methods between 2) and 4). However, productivity is extremely low, and selective synthesis is difficult, which poses a large problem. For this reason, single wall carbon nanotube (SWCNT), fullerene, etc. are very expensive. In addition, the fact that other direct synthetic method of graphene has not been found has become a large obstacle for expanding the application of these materials.
6) Features of manufacturing process of Incubation Alliance Inc. (InALA process)
Incubation Alliance Inc. has been making efforts to develop a new manufacturing method of carbon nanomaterials, aiming at synthesizing them with high productivity and accelerating their applications.
As a result, we have succeeded in the development of high rate CVD process (InALA process) with the characteristics of novelty and high productivity, in cooperation with Prof. Masahiro Toyoda at Oita University, and have been promoting the commercialization of carbon nanotube, graphene, and various carbon nanofibers.
InALA process has a feature to be able to generate CVD in a whole reactor space on simultaneous multiple basis, resulting in a very high productivity.
Figure 2 shows the comparison of the concepts of conventional methods and InALA process regarding the synthesis of carbon nanotube. In the conventional vapor-phase fluidization process, the volume involved in the generation reaction is very small at the edge of a nozzle, so although SWCNT can be easily obtained through the selection of a catalyst, its productivity is low.
On the other hand, according to the conventional substrate CVD method, since CVD proceeds while generating CNT on the substrate supporting the catalyst, a vast area of substrate is required for mass production.
In comparison with these methods, in the case of InALA process, since CVD proceeds in a whole reactor space three-dimensionally and on simultaneous multiple basis, the synthesis of nano carbon with high productivity is possible.
In the cases of graphene and vapor-phase growth graphite, synthesis without catalyst is possible. With the use of a catalyst, it is possible to synthesize with high productivity nearly all the carbon materials including CNT and various carbon nanofibers.
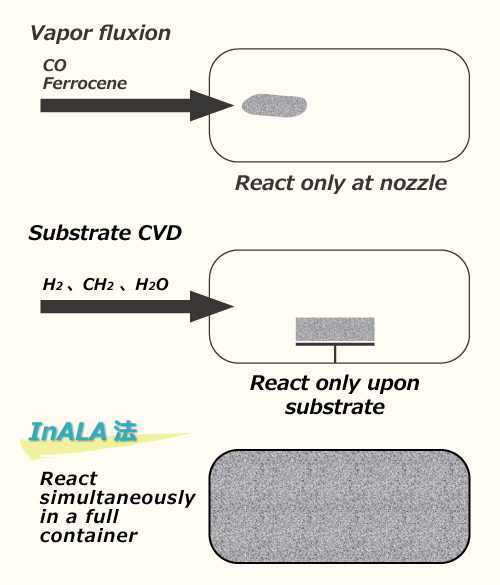
Fig. 2 Comparison between conventional CNT manufacturing methods and InALA process
In Table 1, the comparison between the conventional manufacturing methods for carbon materials and InALA process is shown, with a focus on the generated carbon materials, productivity, and purity. InALA process has high productivity and is able to manufacture high-purity nano carbon, compared with other manufacturing methods. In particular, it can directly synthesize graphene without using substrate and catalyst.
Table 1. Comparison between manufacturing methods
| Manufacturing methods | Sub strate |
Cata- lyst |
Generated carbon material | Produc- tivity |
Purity | ||||
|---|---|---|---|---|---|---|---|---|---|
| Crysta- llinity |
CNT | CNF | Graphene | Full- erene |
|||||
| Solid state graphitization | No | No | ○ | High | Present, Absent | ||||
| Arc discharge | No | No | ○ | ○ | ○ | Low | High | ||
| Conbustion | No | No | ○ | Middle | High | ||||
| CVD (on substrate) |
Yes | Yes | ○ | ○ | Low | Low | |||
| CVD (on substrate) |
Yes | No | ○ | ○ | Low | By base material type | |||
| CVD (Fluidized bed) |
Yes | Yes | ○ | ○ | Middle | Low | |||
| CVD (Veger state fluxion) |
No | Yes | ○ | ○ | Low | Low | |||
| Physical Pealing | No | No | ○ | Low | High | ||||
| Chemical Thin Layer preling | No | No | ○ | Low | High | ||||
| SiC Monocatalyst Sublimation | Yes | No | ○ | Low | Middle | ||||
| InALA | No | No | ○ | ○ | ? | High | High | ||
| InALA | No | Yes | ○ | ○ | ○ | ? | High | High | |